You are here
Media Advisory
Wednesday, April 3, 2024
Drug shows promise for slowing progression of rare, painful genetic disease
NIH-supported clinical trial could lead to first effective treatment for ACDC disease.
What
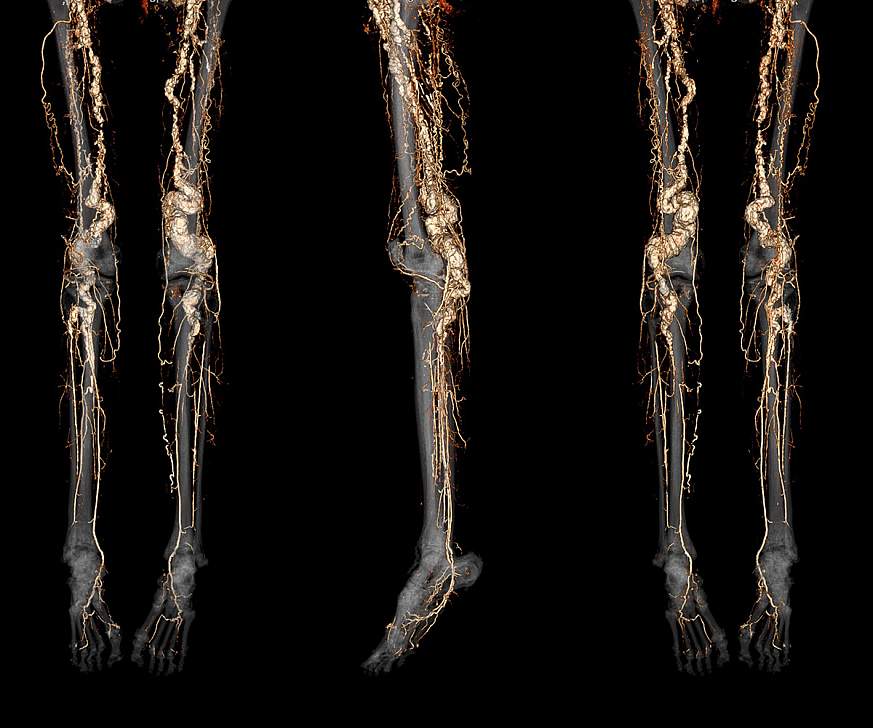
A drug used to treat certain bone diseases shows promise for slowing the progression of a rare, painful genetic condition that causes excessive calcium buildup in the arteries, known as arterial calcification due to deficiency of CD73 (ACDC). These results are from a first-in-human clinical trial supported by the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health. The study, published in the journal Vascular Medicine, could lead to the first effective treatment for the rare disease.
ACDC, which has no known cure, often targets the arteries of the legs and can make walking painful and difficult. It can also affect the joints of the hands, causing pain and deformities. In severe cases, the condition can lead to potential limb loss. Symptoms of the disease often begin in the late teens and 20s. An extremely rare disease, it is believed to affect only about 20 people worldwide and has an estimated prevalence of less than 1 in 1 million. Previous studies have identified the gene for ACDC disease and the biochemical mechanism behind it. More recent studies by the NHLBI research team identified an existing drug, called etidronate, as a potential treatment for ACDC based on disease models in animals and human cells.
In the study, researchers evaluated the safety and effectiveness of etidronate in treating calcification of the arteries and impaired blood flow in the legs of seven people (four women and three men) with ACDC disease. Although few, they collectively represent about one-third of all the known cases in the world. Treatment consisted of taking the oral drug daily for 14 days every three months over a three-year period. The researchers measured calcium deposits using CT scans and tested blood flow using the ankle brachial index, a non-invasive tool, both at the start of the study and as a yearly follow-up after treatment.
Researchers found that etidronate treatments appeared safe, with no adverse side effects reported. The drug appeared to slow the progression of new calcium deposits in the blood vessels of the legs as well as slow the progression of blood flow inhibition. However, the drug did not reverse calcium deposits that were already present in the affected blood vessels and joints, nor did it show clear improvement in blood flow. Questionnaires administered to the patients suggested that symptoms, such as pain and motion impairment, were improved.
Researchers suggest that lessons learned from the current study could allow development of novel therapies for ACDC and larger clinical trials for this disease. The study could also shed light on other diseases involving excessive calcium buildup in the arteries, including peripheral artery disease and atherosclerosis.
Reference
Ferrante EA, Cudrici CD, Rashidi M, et al. Pilot study to evaluate the safety and effectiveness of etidronate treatment for arterial calcification due to deficiency of CD73 (ACDC). Vascular Medicine. 2024. doi: 10.1177/1358863X241235669
Who
Elisa Ferrante, Ph.D., clinical program manager and staff scientist in the Translational Vascular Medicine Branch at NHLBI, and Alessandra Brofferio, M.D., clinical cardiologist with the Laboratory of Cardiovascular Regenerative Medicine of NHLBI.
About the National Heart, Lung, and Blood Institute (NHLBI): NHLBI is the global leader in conducting and supporting research in heart, lung, and blood diseases and sleep disorders that advances scientific knowledge, improves public health, and saves lives. For more information, visit www.nhlbi.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®
