You are here
News Release
Thursday, July 11, 2024
Immunotherapy approach shows potential in some people with metastatic solid tumors
NIH researchers achieved tumor shrinkage in three of seven patients with colorectal cancers.
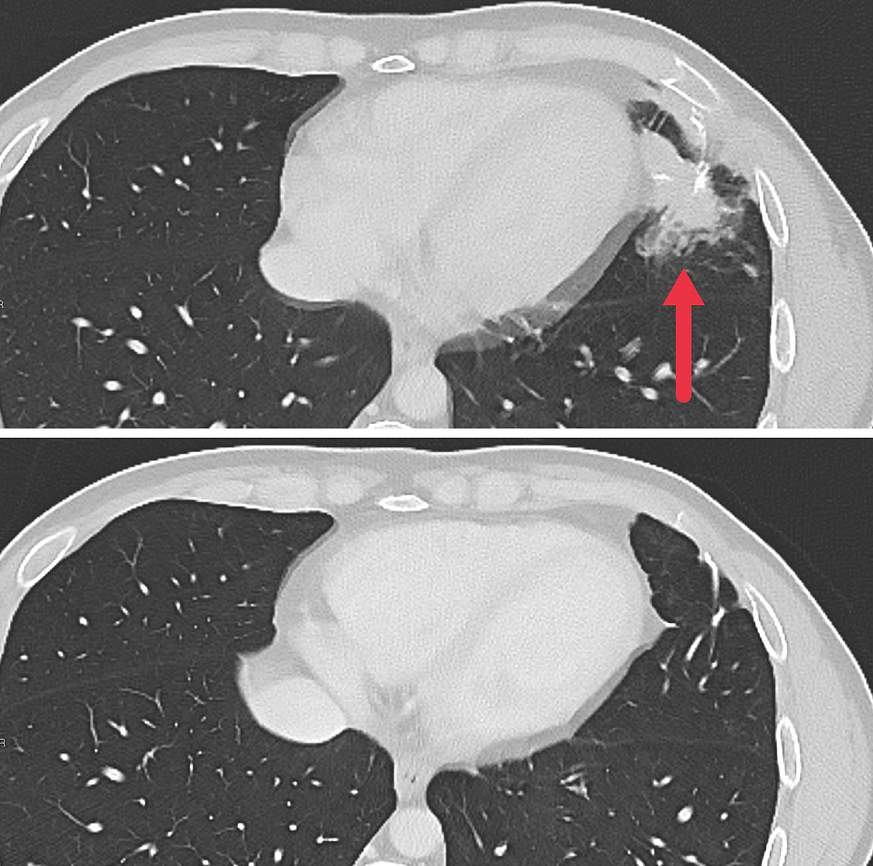
Early findings from a small clinical trial provide evidence that a new cellular immunotherapy approach may be effective in treating metastatic solid tumors. In the trial, researchers from the National Institutes of Health (NIH) genetically engineered normal white blood cells, known as lymphocytes, from each patient to produce receptors that recognize and attack their specific cancer cells. These initial findings are from people with metastatic colorectal cancer who had already undergone multiple earlier treatments. The personalized immunotherapy shrank tumors in some patients and was able to keep the tumors from regrowing for up to seven months. The findings were published July 11, 2024, in Nature Medicine.
One form of cellular immunotherapy, chimeric antigen receptor (CAR) T-cell therapy, has already been shown to be effective against some blood cancers, and another, called tumor-infiltrating lymphocyte (TIL) therapy, has proven to be effective against metastatic melanoma. However, to date, a cellular therapy that’s effective against any other solid cancers has been elusive, according to Steven A. Rosenberg, M.D., Ph.D., of NCI’s Center for Cancer Research (CCR), who co-led the study with Maria Parkhurst, Ph.D., of CCR’s Surgery Branch.
“The fact that we can take a growing metastatic solid cancer and get it to regress shows that the new cellular immunotherapy approach has promise,” Dr. Rosenberg said. “However, it’s important to understand that these findings are preliminary and that the approach needs to be further refined and tested in more types of solid cancers.”
The new approach overcomes two challenges in cellular immunotherapy: how to produce large numbers of T cells that can recognize cancer cells specifically, and how to boost the ability of modified T cells to multiply once they’ve been returned to the patient.
For each patient in the study, Dr. Rosenberg and his colleagues collected lymphocytes present in the patient’s tumors. They then used sophisticated molecular characterization techniques to identify and isolate receptors on those lymphocytes, called T-cell receptors, that recognized specific changes in each patient’s tumor. After genetically sequencing those receptors, they then used a retrovirus to insert the genes for the receptor into normal lymphocytes collected from each patient’s circulating blood.
The genetically modified lymphocytes were then multiplied into the hundreds of millions in the laboratory and infused back into the patients, where they expressed the tumor-specific T-cell receptors and continued to multiply.
“By taking the natural T-cell receptors that are present in a very small number of cells and putting them into normal lymphocytes for which we have enormous numbers—a million in every thimbleful of blood—we can generate as many cancer-fighting cells as we want,” Dr. Rosenberg explained.
As part of a larger phase 2 trial, seven patients with metastatic colon cancer were treated with the experimental personalized cellular immunotherapy. All seven received several doses of the immunotherapy drug pembrolizumab (Keytruda) before the cell therapy and another immunotherapy drug called IL-2 afterward. Three patients had substantial shrinkage of metastatic tumors in the liver, lung, and lymph nodes that lasted for four to seven months. The median time to disease progression was 4.6 months.
Dr. Rosenberg noted that, of the three patients who responded to the treatment, two had received T-cell receptors derived from cytotoxic T cells, which are primarily responsible for killing diseased cells. Dr. Rosenberg said his research team is exploring how to put the T cell receptors into subtypes of normal lymphocytes to improve their reactivity.
Colon cancer is just one of many solid tumors the researchers are studying. The trial is still ongoing and includes patients with different types of solid cancers.
“It's just the very beginning of converting normal lymphocytes into cells capable of treating the common solid cancers,” Dr. Rosenberg said. “What this study shows is that it's possible. Once you know it’s possible, you work to improve it.”
About the National Cancer Institute (NCI): NCI leads the National Cancer Program and NIH’s efforts to dramatically reduce the prevalence of cancer and improve the lives of people with cancer. NCI supports a wide range of cancer research and training extramurally through grants and contracts. NCI’s intramural research program conducts innovative, transdisciplinary basic, translational, clinical, and epidemiological research on the causes of cancer, avenues for prevention, risk prediction, early detection, and treatment, including research at the NIH Clinical Center—the world’s largest research hospital. Learn more about the intramural research done in NCI’s Center for Cancer Research. For more information about cancer, please visit the NCI website at cancer.gov or call NCI’s contact center at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®
References
Parkhurst, M., Goff, S.L., Lowery, F.J. et al. Adoptive transfer of personalized neoantigen-reactive TCR-transduced T cells in metastatic colorectal cancer: phase 2 trial interim results. Nat Med (2024). https://doi.org/10.1038/s41591-024-03109-0
