You are here
May 7, 2024
Antibody reduces risk of malaria in children
At a Glance
- A single dose of a monoclonal antibody given to children 6 to 10 years of age in Mali proved up to 77% effective at preventing malaria disease.
- Researchers plan to test the antibody in other populations at high risk of death from malaria, including infants and pregnant women.

More than 600,000 people worldwide die from malaria every year, and most are children in Africa. A parasite called Plasmodium, which is spread by mosquitos, causes the disease. Most cases and deaths are caused by the species P. falciparum.
Malaria-prevention strategies include spraying insecticides and administering drugs to help prevent disease. But these are hampered by emerging resistance to insecticides and anti-malarial drugs. The use of preventive drugs also requires frequent contacts with health care providers, which can limit their use in low-resource countries. The recent approval of two new malaria vaccines is a major milestone, but their efficacy is limited and short-lived.
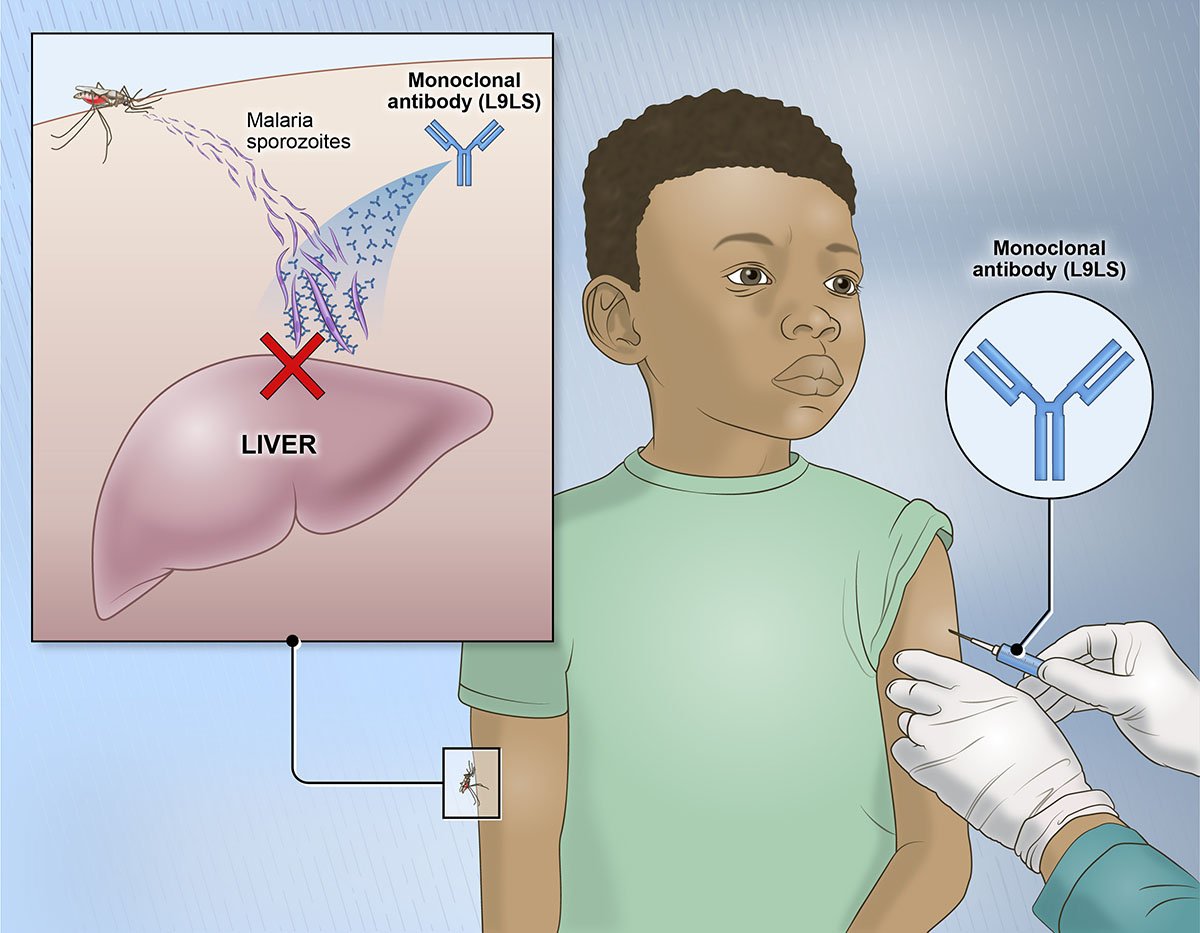
NIH researchers have been working to develop laboratory-made antibodies called monoclonal antibodies to help prevent malaria infections. One, called L9LS, was based on an antibody isolated from a volunteer who received an experimental malaria vaccine. The researchers engineered this candidate antibody to make it last longer in the body.
L9LS binds to a specific protein on the surface of P. falciparum sporozoites, the parasite stage that is transmitted from mosquitos to humans. L9LS binding prevents sporozoites from infecting cells in the liver. This is a necessary step for parasites before infecting blood cells and spreading to other mosquitoes through blood feeding.
In 2022, a research team at NIH’s Vaccine Research Center found that L9LS protected 15 of 17 (88%) adults who had never had malaria from a controlled P. falciparum infection. In a new study, researchers from NIH and the University of Bamako in Mali tested the antibody in children over a six-month malaria season. The results were published on April 26, 2024, in the New England Journal of Medicine.
The study took place in Mali, where malaria transmission is extremely high from July to December. The team first tested three different doses of the antibody in adults. This was followed by two different doses in children. When no serious side effects were seen, an additional 225 children joined the study to test whether the antibody could prevent malaria infection and disease.
The children received a single injection with one of two doses of L9LS or a placebo at the beginning of the malaria season. Over the next six months, only 19% of the children who received the higher dose of the antibody and 28% who received the lower dose developed clinical symptoms of malaria. In contrast, almost 60% of those who received the placebo developed symptoms of malaria. The high dose, then, was 77% effective at preventing malaria disease.
L9LS also prevented malaria infections: 40% of those who received the higher dose and 48% of those who received the lower dose had evidence of the parasite in their blood. In contrast, 81% of those who received the placebo had evidence of the parasite. Side effects from injection of the antibody were mild and resolved on their own in all groups.
“A long-acting monoclonal antibody delivered at a single health care visit that rapidly provides high-level protection against malaria in these vulnerable populations would fulfill an unmet public health need,” says Dr. Jeanne Marrazzo, director of NIH’s National Institute of Allergy and Infectious Diseases.
An ongoing clinical trial in Kenya is now testing the antibody in children 5 months to 5 years of age. Researchers are also preparing to test whether the antibody can safely prevent malaria during pregnancy.
Related Links
- Skin Compounds Associated with Attractiveness to Mosquitoes
- Antibody Treatment Protects Adults Against Malaria
- How Mosquitoes Distinguish People from Animals
- Monoclonal Antibody Prevents Malaria in Early Trial
- Malaria Vaccines Provide Strong and Lasting Immunity
- Universal Mosquito Vaccine Tested
- Engineering Malaria Resistance in Mosquitoes
- Drug Prevents Malaria in High-Risk Region
- Battling Bites: Blocking Mosquito-Borne Diseases
- Malaria
References: Subcutaneous Administration of a Monoclonal Antibody to Prevent Malaria. Kayentao K, Ongoiba A, Preston AC, Healy SA, Hu Z, Skinner J, Doumbo S, Wang J, Cisse H, Doumtabe D, Traore A, Traore H, Djiguiba A, Li S, Peterson ME, Telscher S, Idris AH, Adams WC, McDermott AB, Narpala S, Lin BC, Serebryannyy L, Hickman SP, McDougal AJ, Vazquez S, Reiber M, Stein JA, Gall JG, Carlton K, Schwabl P, Traore S, Keita M, Zéguimé A, Ouattara A, Doucoure M, Dolo A, Murphy SC, Neafsey DE, Portugal S, Djimdé A, Traore B, Seder RA, Crompton PD; Mali Malaria mAb Trial Team. N Engl J Med. 2024 May 2;390(17):1549-1559. doi: 10.1056/NEJMoa2312775. Epub 2024 Apr 26. PMID: 38669354.
Funding: NIH’s National Institute of Allergy and Infectious Diseases (NIAID).