You are here
June 11, 2019
Technique lowers risk of complications in heart valve replacement
At a Glance
- Researchers devised a technique to avoid a fatal complication during a procedure to replace the heart’s mitral valve.
- The technique may increase treatment options for people who are too elderly or frail to have open heart surgery for valve replacement.
The heart has valves that control the flow of blood in and out of its chambers. The mitral valve controls the flow of blood into the lower left chamber, or ventricle, which is the heart’s main pumping chamber. When disease causes this valve to narrow—a condition called stenosis—blood flow is partially blocked. A diseased valve can also leak and cause regurgitation, in which blood flows back through the valve.
People with mitral valve stenosis or regurgitation may feel tired, have chest pain, and be short of breath. Over time, people with mitral valve disease may develop an enlarged heart and heart failure.
Treatment includes valve repair and replacement. Surgeons can perform these procedures through open heart surgery. For older or frail people who can’t have open heart surgery, another option is a transcatheter mitral valve replacement (TMVR). It involves threading a long, thin tube, called a catheter, through an artery in the leg to the heart to replace the damaged mitral valve.
The mitral valve consists of two leaflets of tissue. Unfortunately for many patients who get TMVR, a deadly complication can develop when a leaflet is pushed back and blocks blood flow. This blockage is known as left ventricular outflow tract obstruction.
To increase the success of TMVR, a team of researchers led by Drs. Jaffar M. Khan and Robert J. Lederman at NIH’s National Heart, Lung, and Blood Institute and Dr. Vasilis C. Babaliaros at Emory University Hospital developed a novel technique. It’s called LAMPOON for intentional “laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction.” This technique is similar to their BASILICA technique, which is used to split an aortic valve leaflet.
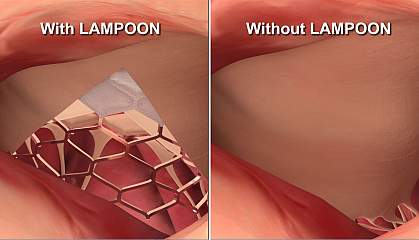
For LAMPOON, the doctor inserts two catheters through a blood vessel in the patient’s groin and into the heart. The doctor then uses an electrified wire within the catheter to split open the leaflet. At that point, patients have TMVR. Results were published in the Journal of the American College of Cardiology on May 28, 2019.
Between June 2017 and June 2018, the research team tested the technique in 30 people who were too frail to be treated with open heart surgery and at high risk of obstruction from TMVR. All patients survived the procedure, and nearly all (93%) survived 30 days. Other methods have a 30-day survival rate of about 38%. About 73% of the patients had a successful LAMPOON followed by successful TMVR without the need for further intervention.
“When [surgeons] replace valves and cut open the heart, they see the leaflet and cut it out,” Khan says. “LAMPOON is designed for patients who need a new mitral valve, but can’t, or may not want to undergo open heart surgery.”
The researchers are monitoring the patients to track the long-term success of this experimental method. LAMPOON may increase treatment options for high-risk patients who weren’t previously eligible for mitral heart valve procedures.
Related Links
- Procedure Makes Heart Valve Replacement Safer for High-Risk Patients
- Heart Bypass Surgery Brings Long-Term Benefits
- MRI Shows Promise for Heart Procedures
- Cardiac Catheterization
- Heart Valve Disease
References: Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, Paone G, Eng MH, Leshnower BG, Gleason PT, Chen MY, Wang DD, Tian X, Stine AM, Rogers T, Lederman RJ. J Am Coll Cardiol. 2019 May 28;73(20):2521-2534. doi: 10.1016/j.jacc.2019.02.076. Erratum in: J Am Coll Cardiol. 2019 May 25. PMID: 31118146.
Funding: NIH’s National Heart, Lung, and Blood Institute; Emory University; Henry Ford Hospital; Carilion Roanoke Memorial Hospital; Inova Fairfax Hospital; University of Washington; Edwards Lifesciences.

