You are here
October 20, 2014
Developing Insulin-Producing Cells to Treat Diabetes
At a Glance
- Researchers designed a protocol to transform human stem cells into pancreatic beta cells that produce insulin and respond to glucose.
- The finding could lead to new stem cell-based therapies to treat diabetes.
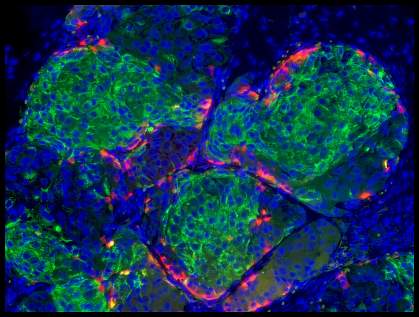
Diabetes is a disorder in the use of glucose, a sugar that serves as fuel for the body. When blood glucose levels rise, beta cells in the pancreas normally make the hormone insulin. Insulin triggers cells throughout the body to take up sugar from the blood. In type 2 diabetes, the most common form, tissues in the body lose their sensitivity to insulin, and pancreatic beta cells can’t make enough insulin to keep glucose levels in check. In type 1 diabetes, the body’s own immune system attacks and destroys beta cells. High blood glucose levels can lead to heart disease, blindness, and other health problems over time.
One strategy to treat diabetes is to replace destroyed beta cells. Transplanted human pancreatic cells from deceased donors have been successfully used to treat people with type 1 diabetes. However, this approach is limited by the availability of donor cells and the side effects of immunosuppression.
Another approach would be to develop functioning beta cells from stem cells. Stem cells have the potential to transform into many different cell types. Human pluripotent stem cells are adult cells that have been genetically reprogrammed to take on the characteristics of embryonic stem cells. They can grow indefinitely in the laboratory and can theoretically change, or differentiate, into any cell type found in the body.
A team led by Dr. Douglas Melton at Harvard University set out to transform stem cells into beta cells that could replace damaged beta cells. Although scientists have been able to convert stem cells into insulin-producing cells, these cells don’t have markers that indicate they are beta cells, and they aren’t responsive to glucose. The new study was funded in part by NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Results appeared online on October 9, 2014, in Cell.
The team grew a human embryonic stem cell line and 2 human-induced pluripotent stem cell lines in a culture system that enabled them to produce large numbers of cells. They tested more than 150 combinations of over 70 compounds to tease out a method to produce functional human beta cells from the cultured stem cells. They eventually fine-tuned a protocol that involved 5 different growth media and 11 different factors. When added in exact combinations over a period of several weeks, they transformed human pluripotent stem cells into beta cells that functioned similarly to normal adult beta cells.
The cultured beta cells had markers found on normal beta cells, showed changes in calcium levels when exposed to glucose, and packaged insulin into granules. When transplanted into mice, they secreted insulin in response to glucose. When the cells were transplanted into diabetic mice, abnormally high blood glucose levels lowered.
“The biggest hurdle has been to get to glucose-sensing, insulin-secreting beta cells, and that’s what our group has done,” says Melton. “We are now just one preclinical step away from the finish line.”
Additional work will be needed to develop these cells for clinical use. They could also serve as a useful screening tool for diabetes drugs.
—by Carol Torgan, Ph.D.
Related Links
- Diabetes
- Diabetes HealthSense
- Stem Cell Therapy Rebuilds Heart Muscle in Primates
- Stem Cells Form Light-Sensitive 3-D Retinal Tissue
- Stem Cell Information
References: Generation of Functional Human Pancreatic β Cells In Vitro. Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Cell. 2014 Oct 9;159(2):428-39. doi: 10.1016/j.cell.2014.09.040. PMID: 25303535.
Funding: NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Harvard Stem Cell Institute, Juvenile Diabetes Research Foundation, Helmsley Charitable Trust, JPB Foundation, M. and A. Barry, and the Howard Hughes Medical Institute.
