You are here
June 4, 2024
Adrenal cell affects parental behavior in mice
At a Glance
- Researchers traced differences in parental care between two mouse species to a new cell type in the adrenal glands.
- The findings shed light on how the evolution of new cell types outside the brain can affect social behaviors.
Specialized cells can help regulate certain animal behaviors, such as mating, maternal aggression, and parental care. But often, how these cell types evolve and how they affect behavior are not well understood.
The oldfield mouse and the prairie deer mouse are closely related, yet the former is monogamous while the latter is promiscuous. A research team led by Dr. Andres Bendesky at Columbia University took advantage of this difference to explore the basis of monogamous behavior in oldfield mice. Results from the NIH-funded study appeared in Nature on May 15, 2024.
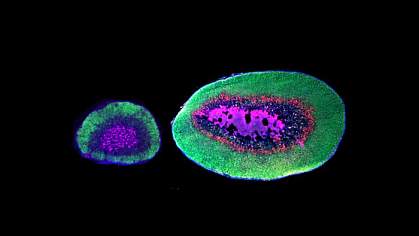
The researchers noticed that oldfield mice had adrenal glands more than six times heavier than those of deer mice relative to their body weight. Steroid hormones produced in the adrenal glands can influence animal behaviors. So, the team compared gene activity in the adrenal glands in both species.
They found that one particular gene, Akr1c18, was about 3,200 times more active in oldfield mice than in deer mice. This gene encodes an enzyme that converts the hormone progesterone into 20α-hydroxyprogesterone (20α-OHP). As a result, oldfield mice had much more 20α-OHP in their adrenal glands and blood than deer mice. In key parental care regions of the brain, 20α-OHP was processed into another compound that can affect neuronal signals.
Injecting oldfield mouse parents with 20α-OHP promoted parental behaviors typical of monogamous species. The mice given 20α-OHP groomed and huddled with their pups more, retrieved more unfamiliar pups to their nests, and built more robust nests.
An analysis of gene activity in individual adrenal cells revealed a class of cells in oldfield mice that was absent in deer mice. These cells formed a distinct layer within the adrenal gland, which the team named the zona inaudita. Zona inaudita cells featured high activity of Akr1c18, as well as genes for certain proteins that make up the extracellular matrix and proteins that control the activity of other genes.
To find out how these zona inaudita cells might have evolved, the researchers crossbred oldfield mice and deer mice, and then crossbred their offspring. The resulting mice carried varying mixtures of the two species’ genomes. The team found two genomic regions where having the oldfield versions enhanced the activity of a nearby gene. This, in turn, increased the activity of other zona inaudita genes. The findings suggest that genetic variation affecting the activity of two key genes led to the evolution of zona inaudita cells in oldfield mice.
To pinpoint when adrenal production of 20α-OHP evolved, the team measured Akr1c18 levels in two additional related mouse species. They estimated that this ability evolved fairly recently, within about the last 20,000 years. Higher Akr1c18 levels led to more 20α-OHP, which in turn promoted parental behaviors typical of monogamous species. The results provide insight into how new cell types can evolve outside the brain to affect animal behavior.
“I hope that our study motivates further investigation into the link between 20⍺-OHP and parenting in humans,” says co-author Dr. Jennifer R. Merritt. “We have so much to learn about the role this hormone plays in human parental behavior.”
—by Brian Doctrow, Ph.D.
Related Links
- How the Black Death Shaped Human Evolution
- Understanding How the Brain Tracks Social Status and Competition
- Cerebellum Connects to Brain’s Reward System
- New Brain Network Identified for Social Interactions
- Anxiety Molecules Affect Male and Female Mice Differently
- Do Social Ties Affect Our Health? Exploring the Biology of Relationships
References: Evolution of a novel adrenal cell type that promotes parental care. Niepoth N, Merritt JR, Uminski M, Lei E, Esquibies VS, Bando IB, Hernandez K, Gebhardt C, Wacker SA, Lutzu S, Poudel A, Soma KK, Rudolph S, Bendesky A. Nature. 2024 May 15. doi: 10.1038/s41586-024-07423-y. Online ahead of print. PMID: 38750354.
Funding: NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Institute of General Medical Sciences (NIGMS); Searle Scholars Program; Esther A. & Joseph Klingenstein Fund; Simons Foundation Autism Research Initiative; Alfred P. Sloan Foundation; Canadian Institutes of Health Research; Albert Einstein College of Medicine.
