You are here
June 4, 2012
Clues to Alzheimer’s Disease
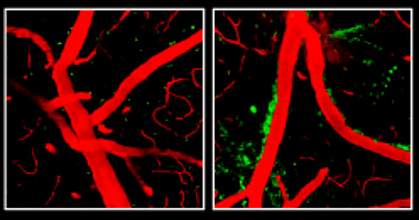
Researchers may have discovered a mechanism behind the largest known genetic risk factor for late-onset Alzheimer's disease. The finding suggests possible strategies for prevention as well as a potential new drug target.
Alzheimer's disease is the most common cause of dementia in older adults, affecting more than 5 million Americans. A hallmark of the disease is a protein fragment called beta-amyloid, which is thought to be toxic and forms clumps, or plaques, within the brain. Past studies have revealed several genetic risk factors for Alzheimer's disease. A gene called APOE has shown the strongest connection to the most common, late-onset form of the disease.
APOE encodes a protein that helps regulate the levels and distribution of cholesterol and other lipids in the body. The gene exists in 3 forms. APOE2 is thought to play a protective role against both Alzheimer's and heart disease, APOE3 is believed to be neutral, and APOE4 confers a higher risk for both conditions. Outside the brain, the APOE4 protein appears to be less effective than the other versions at clearing away cholesterol. How it contributes to Alzheimer's disease in the brain, however, has been a mystery.
A research team led by Dr. Berislav Zlokovic of the University of Southern California approached the question by studying several lines of genetically engineered mice. One lacks the mouse version of the APOE gene altogether, and 3 other lines produce only human APOE2, APOE3 or APOE4. The study was funded by NIH's National Institute of Neurological Disorders and Stroke (NINDS) and National Institute on Aging (NIA). It appeared in the early online edition of Nature on May 16, 2012.
The researchers discovered that mice whose bodies made only APOE4, or no APOE at all, had a leaky blood-brain barrier. The blood-brain barrier includes a network of cells that line tiny blood vessels in the brain. Normally, this barrier allows nutrients to pass from circulating blood into the brain while keeping harmful substances out. With the barrier compromised, harmful proteins in the blood made their way into the mice's brains. After several weeks, the researchers were able to detect a loss of small blood vessels, changes in brain function, and a loss of connections between brain cells.
The researchers found that APOE2 and APOE3 help control the levels of an inflammatory molecule called cyclophilin A (CypA), but APOE4 does not.Levels of CypA were raised about 5-fold in the blood vessels of mice that produce only APOE4. The excess CypA activated an enzyme called MMP-9, which destroys parts of the blood-brain barrier.
Treating the mice with the immunosuppressant drug cyclosporine A, which inhibits CypA, preserved the integrity of the blood-brain barrier and lessened damage to the brain. An inhibitor of the MMP-9 enzyme had similar effects.
These findings identify CypA as a potential new drug target for Alzheimer's disease. They also suggest that one approach to preventing Alzheimer's disease among APOE4 carriers may be to work on improving vascular health.
The connection between APOE4 and beta-amyloid remains unclear. Damage caused by APOE4 may make it harder to clear beta-amyloid from the brain. “Understanding the role of APOE4 in Alzheimer's disease may be one of the most important avenues to a new therapy,” Zlokovic says.
Related Links
References: Nature. 2012 May 16;485(7399):512-6. doi: 10.1038/nature11087. PMID: 22622580
