You are here
May 4, 2015
Devices Assess Cancer Drugs in Tumors
At a Glance
- Two devices, developed independently, can gauge how tumors will respond to various drugs.
- With further development, these technologies could help doctors determine the ideal treatment for each person’s particular cancer.
Although numerous drugs are available to treat cancers, determining which ones to use can be challenging. As tumors grow, they often become resistant to treatments. Cancer cells mutate quickly, which can help them adapt and survive. Other factors are also involved in resistance, including various aspects of the tumor’s microenvironment. Because these complex factors are challenging to recreate outside of the body, it’s difficult to test and predict how patient’s tumors will respond to different treatments.
Two separate research groups set out to address this problem by creating tiny devices that can test how tumors within a patient respond to various drugs. Both teams were funded in part by NIH’s National Cancer Institute (NCI). Papers describing the studies were published together on April 22, 2015, in Science Translational Medicine.
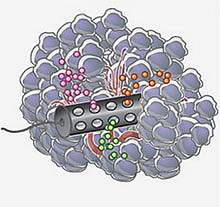
A team led by Drs. Oliver Jonas and Robert Langer at the Massachusetts Institute of Technology developed a small device — only 4 mm long and 820 μm in diameter — that can be implanted using a standard biopsy needle. The device contains reservoirs that release tiny doses of up to 16 individual drugs or drug combinations into distinct regions. After 24 hours, a second biopsy needle is used to retrieve the device, along with a small column of tissue around it. The tissue is then examined to assess the effects of the drugs on the tumor.
Using established mouse models of human melanoma, breast cancer, and prostate cancer, the researchers demonstrated that the device could accurately predict how effective treatments would be when used system-wide. Based on these results, the scientists are planning to launch a clinical trial in breast cancer patients next year.
“The approach that we thought would be good to try is to essentially put the lab into the patient,” Jonas says. “It’s safe and you can do all of your sensitivity testing in the native microenvironment.”
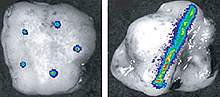
Another device was developed by a team led by Dr. Jim Olson at Fred Hutchinson Cancer Research Center and Dr. Richard Klinghoffer at Presage Biosciences, a company founded by Olson. Called CIVO, the device features an array of up to 8 needles that can inject small amounts of candidate drugs directly into tumors. The drugs are delivered as the needles are retracted, leaving column-like tracks of the drugs though the tumor. After 24-72 hours, tumor samples are taken and processed. The tumor’s response to each drug is assessed with a sophisticated image analysis system.
The researchers tested the microinjection system in mouse models of human lymphomas and lung cancers, as well as in canines. The cellular changes around the drug tracks predicted the animals’ response to the drugs. A screen of 97 compounds in mice also revealed a new anti-cancer agent for a type of resistant tumor. These results show that CIVO could potentially be used to identify drugs to treat resistant tumors.
The scientists tested CIVO in a clinical setting in 4 human lymphoma patients. No serious adverse events were reported in the small pilot study. Ongoing testing of the system now continues in a clinical trial.
“This sets the stage for a new type of pre-phase 1 clinical study in which multiple drugs or drug combinations can be tested simultaneously, directly in a patient’s own tumor, without toxicity associated with systemic drug delivery,” Klinghoffer says.
— by Harrison Wein, Ph.D.
Related Links
- Isolated Cancer Cells May Lead to Personalized Treatments
- NIH Health Information: Cancer
- Milestones in Cancer Research & Discovery
- Clinical Trial of CIVO Microdosing System
References: An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Jonas O, Landry HM, Fuller JE, Santini JT Jr, Baselga J, Tepper RI, Cima MJ, Langer R. Sci Transl Med. 2015 Apr 22;7(284):284ra57. doi: 10.1126/scitranslmed.3010564. PMID: 25904741.
Funding: NIH’s National Cancer Institute (NCI); Kibur Medical; Presage Biosciences; and Seattle Children’s Hospital Neuro-Oncology Fund.
