You are here
July 30, 2012
HIV Protein Strikes a Fleeting Pose
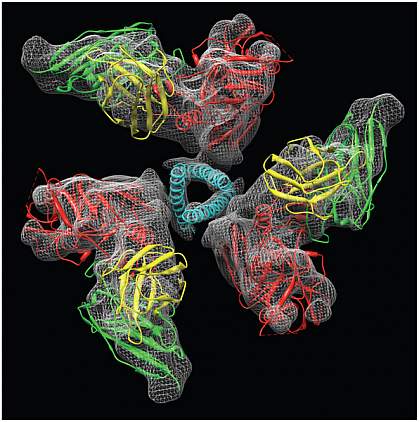
NIH scientists have discovered a new intermediate structure of the HIV entry protein. This temporary structure, which occurs just before HIV infects a cell, may offer a potential target for developing an HIV vaccine.
HIV, the virus that causes AIDS, affects more than 30 million people worldwide, and millions more become infected each year. Once in the body, HIV invades immune cells known as T cells by latching onto a receptor and a co-receptor on the cell's surface. T cells are responsible for fighting off viruses—including HIV itself.
Despite recent advances in treatment, scientists have not yet designed a vaccine that protects people from HIV. One challenge is that the proteins on the viral surface—the HIV envelope glycoproteins (Env)—mutate rapidly, changing their shape and evading the immune system. Only the deep inner portions of Env remain constant, and these are generally hidden from the immune system by the glycoprotein's structure.
Over the last 25 years, scientists have visualized the structure of Env and uncovered details of the HIV entry mechanism. But much less is known about the steps between Env binding to the T-cell receptor known as CD4 and the point at which the virus fuses with the cell. In a study published in PLoS Pathogens on July 12, 2012, a team of scientists led by Dr. Sriram Subramaniam of NIH's National Cancer Institute (NCI) set out to investigate the structural changes of Env during this early stage of HIV infection.
The researchers used a technique called cryo-electron microscopy to visualize the native structure of Env. In this technique, the whole virus or purified viral proteins are frozen so rapidly that the ice stays liquid-like. This specialized frozen state keeps the proteins in their natural, life-like positions as they're imaged under the electron microscope, which can reveal the structure of molecules at high resolution.
The researchers first obtained a 3-D structure of Env alone. They then compared that structure with the glycoprotein bound either to the CD4 receptor or to an antibody that mimics the co-receptor. The structures revealed that when Env is bound by either of the receptors, the glycoprotein opens up into an activated state, in which 3 helices spring out at the center of Env. This novel activated intermediate lies midway between the inactive (unbound) position and the next known structure of Env, which occurs just after fusion. The finding offers a look at one of the first steps in the HIV fusion process.
The scientists also determined the structure of Env bound to a neutralizing antibody, VRC01, which is known to block many strains of HIV from infecting human cells. This antibody, the researchers found, keeps Env in its inactive state and prevents the glycoprotein from binding to its receptors.
“We now have an improved understanding of how different neutralizing antibodies work,” says Subramaniam. “What we also have is a unique snapshot of the surface of HIV just as it is about to infect a cell. We anticipate finding more of these intermediates as we continue our work, and each will likely provide invaluable information for designing effective HIV immunogens and vaccines.”
—by Lesley Earl, Ph.D.
Related Links
- Antibodies Protect Human Cells from Most HIV Strains
- Making Antibodies that Neutralize HIV
- "Entry Claw" Helps the HIV Virus Infect Host Cells
- AIDS information
References: PLoS Pathog. 2012 Jul;8(7):e1002797. Epub 2012 Jul 12. PMID: 22807678
