You are here
November 10, 2014
Immune Cells in Heart Help it Mend
At a Glance
- Researchers found that the hearts of mice have resident macrophages, a type of immune cell, that play a key role in recovery from damage.
- The findings suggest that therapies targeting specific groups of macrophages might serve as potential treatment for heart failure.
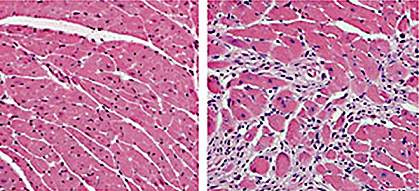
When tissues become injured, the body mounts an inflammatory response. This serves to remove damaged tissue and to aid in repair. Macrophages are a type of immune cell that play a key role in inflammation. These cells are found in the bloodstream and in tissues in the body.
Recent research has shown that macrophages located in tissues such as the heart have an embryonic lineage. This makes them distinct from macrophages in the bloodstream, which originate from monocytes, a type of white blood cell.
Scientists have long been working to understand how inflammation, which is essential for tissue repair, can also be harmful after an injury. A team led by Drs. Kory J. Lavine and Douglas L. Mann at the Washington University School of Medicine set out to determine whether the different populations of macrophages might play distinct roles in inflammation and tissue repair.
The heart of a newborn has a much greater capacity for tissue repair than the heart of an adult. The scientists thought this might be due to different populations of macrophages in newborn versus adult hearts. They developed a model of heart injury in mice using genetics that enabled them to induce cell death in a precise fashion at different developmental stages. The research was funded in part by NIH’s National Heart, Lung, and Blood Institute (NHLBI). Results appeared online on October 27, 2014, in Proceedings of the National Academy of Sciences.
The researchers found that when they induced heart damage, there was an increase in the number of macrophages in both newborn and adult mice. However, there was a difference in macrophage composition. Newborn mice had an increase in embryo-derived heart macrophages. These macrophages produced very little inflammation, and when tested in cell culture assays, promoted blood vessel growth and the formation of new heart cells. The resident macrophages helped heart function recover following injury by promoting tissue repair, rather than inflammation.
The healthy hearts of adult mice also contained embryonic-derived macrophages with similar properties. However, following heart injury, these immune cells were replaced by monocytes and macrophages from the circulation that promoted inflammation rather than repair.
By inhibiting the movement of monocytes from the circulation into injured adult mouse hearts, the researchers preserved the population of embryonic-derived macrophages, decreased inflammation, and improved the heart’s ability to repair.
These findings indicate that the heart’s own macrophages play a key role in recovery of the injured heart. Therapeutics targeting specific groups of macrophages may thus serve as potential treatment for conditions involving tissue damage, such as heart failure.
“We have identified similar immune cell subtypes that are present in the human heart,” Lavine says. “We need to find out more about their roles in heart failure in patients and understand more about how macrophages that reside in the heart promote repair.”
—by Carol Torgan, Ph.D.
Related Links
- Molecule Protects Against Heart Failure in Mice
- Stem Cell Therapy Rebuilds Heart Muscle in Primates
- Immune System
- Heart Failure
References: Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Proc Natl Acad Sci U S A. 2014 Oct 27. pii: 201406508. [Epub ahead of print]. PMID: 25349429.
Funding: NIH’s National Heart, Lung, and Blood Institute (NHLBI); the Oliver Langenberg Physician-Scientist Training Program; and the Washington University Center for the Investigation of Membrane Excitability Diseases Live Cell Imaging Facility.
