You are here
April 28, 2020
Lab-made eye cells restore vision in mice
At a Glance
- Researchers reprogrammed skin cells into light-sensing eye cells that restored sight in mice.
- The technique may lead to new approaches for modeling and treating eye diseases.
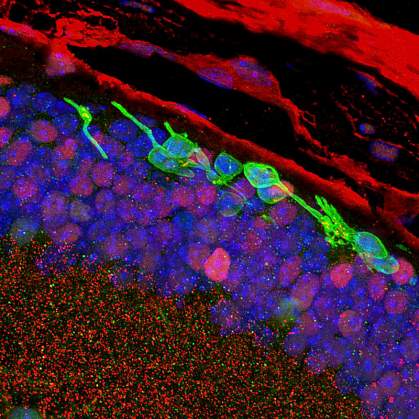
The retina is a light-sensitive layer of tissue at the back of the eye. When light hits the retina, special cells called photoreceptors turn the light into electrical signals. Some eye diseases like macular degeneration can lead to damage and ultimately loss of the retina’s photoreceptors, causing permanent vision loss.
Stem cell therapy is a promising technique to replace dying photoreceptors. Researchers can create stem cells from skin or blood—called induced pluripotent stem cells—and then program them to become photoreceptors in the eye. These lab-made cells may then be transplanted into the retina to potentially restore sight. However, this process is difficult and time-consuming.
Researchers led by Dr. Sai Chavala at CIRC Therapeutics, Inc., Texas Christian University, and the University of North Texas Health Science Center set out to directly reprogram skin cells into photoreceptors, eliminating the need for stem cells. The study was funded in part by NIH’s National Eye Institute (NEI) and also involved NEI investigators. Findings were published in Nature on April 15, 2020.
The team began by testing a combination of four small compounds known to convert skin cells into neurons. By modifying this mixture and then adding another compound reported to reprogram cells into photoreceptors, they were able to chemically transform the skin cells into ones similar to rod photoreceptors. Rods work at low levels of light and are important for night vision.
The lab-made rod-like cells looked and functioned similarly to rods found naturally in the eye. The genes expressed by the new cells were also similar to those in real rod photoreceptors.
The researchers next transplanted the cells into mice with retinal degeneration. They tested their pupillary reflex, which controls the diameter of the pupil in response to light. In low light, pupil constriction depends on functioning rods. Within a month of transplanting the cells, six of 14 mice showed strong pupil constriction under low light, compared to none of the untreated controls.
Mice tend to seek out safe, dark locations, which requires vision in low light. The treated mice with restored pupil reflexes were significantly more likely to seek out and spend time in dark spaces than either untreated controls or treated mice without pupil constriction.
Three months after transplantation, the lab-made photoreceptors were still alive and integrated into the mouse retina.
The new technique is considerably more efficient than current approaches using induced pluripotent stem cells. Those can take six months before cells or tissues are ready for transplantation. In comparison, the reprogramming used in this study turned skin cells into functional rods ready for transplant in only 10 days.
“This is the first study to show that direct, chemical reprogramming can produce retinal-like cells, which gives us a new and faster strategy for developing therapies for age-related macular degeneration and other retinal disorders caused by the loss of photoreceptors,” says senior NEI investigator Anand Swaroop.
Related Links
- Patch Replaces Damaged Retinal Cells
- Regenerating Retinal Cells in Mice
- Genetic Engineering Prevents Retinal Cell Loss in Mice
- Novel Gene-Editing Method Improves Vision in Blind Rats
- Long-Term Benefits of Age-Related Macular Degeneration Treatments
- The Genetics of Age-Related Macular Degeneration
- Retinal Device Restores Sight in Mice
- Age-Related Macular Degeneration
References: Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Mahato B, Kaya KD, Fan Y, Sumien N, Shetty RA, Zhang W, Davis D, Mock T, Batabyal S, Ni A, Mohanty S, Han Z, Farjo R, Forster M, Swaroop A, Chavala SH. Nature. 15 April 2020, http://dx.doi.org/10.1038/s41586-020-2201-4.
Funding: NIH’s National Eye Institute (NEI); Nancy Lee and Perry R. Bass Endowment; Foundation Fighting Blindness.
