You are here
October 17, 2017
Mechanisms of age-related bone loss
At a Glance
- Experiments in mice revealed mechanisms that may help explain why bones become weaker in older adults.
- A better understanding of these processes will inform strategies to develop novel therapies to reduce age-related bone loss.
Bone is comprised of a mineral and protein scaffold filled with bone cells. This structure is continually broken down and renewed. When the rate of bone loss outpaces the rate of replacement, bones weaken, eventually leading to a condition known as osteoporosis. Many factors can contribute to osteoporosis, including aging, certain medications, and hormonal changes.
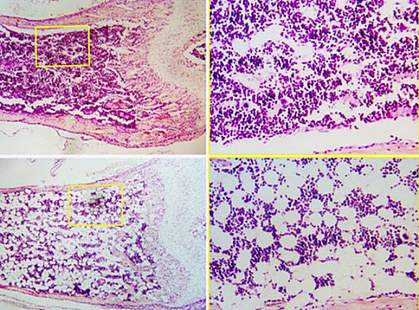
Osteoblasts, the cells that build bone, are derived from mesenchymal stem cells in the bone marrow. These skeletal stem cells can also give rise to other types of cells, including fat cells. The bone marrow of older adults has fewer bone-building osteoblasts and more fat cells than that of younger people. The mechanisms responsible for these changes, however, are unknown.
A research team led by Drs. Yi-Ping Li and Wei Chen at the University of Alabama at Birmingham has been studying the signals that determine whether marrow mesenchymal stem cells develop, or “differentiate,” into osteoblasts or fat cells. In past work, the team found that a protein called Cbfβ is involved in osteoblast differentiation. Cbfβ is also involved in skeletal development and fracture healing.
In the current study, the team explored how Cbfβ affects marrow stem cell differentiation in mice. They deleted the Cbfβ gene at three different stages of osteoblast development: in mesenchymal stem cells, an intermediate stage, and early osteoblasts. The work was funded by NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and National Institute of Dental and Craniofacial Research (NIDCR). Results appeared in Proceedings of the National Academy of Sciences on September 19, 2017.
Cbfβ deficiency at all three stages of differentiation reduced bone density in the mice and dramatically increased their bone marrow fat content. Further testing confirmed that there were more fat cells in the bone marrow of the Cbfβ-deficient mice than the control mice. The bones of the Cbfβ-deficient mice resembled that of aged control mice. Cbfβ levels were also dramatically lower in the aged control mice than in younger control mice. These results suggest that a drop in Cbfβ could contribute to the age-related shift from osteoblast to fat cell production.
A series of lab experiments confirmed that, without Cbfβ, cells at any stage of osteoblast differentiation could switch to form fat cells. Cbfβ inhibits fat cell formation through an important cell signaling pathway called Wnt/β-catenin. It also inhibits expression of a gene that regulates adipose cell formation called c/ebpα. The team showed that Cbfβ plays a critical role in maintaining osteoblast lineage through both these mechanisms.
“Our data detail the underlying pathways that cause progenitor cells and early osteoblasts to create fat cells instead of bone-producing cells,” Li says. “They also suggest that maintaining Cbfβ might be an effective way to prevent age-associated osteoporosis in people.” However, this idea still needs to be tested in humans.
—by Harrison Wein, Ph.D.
Related Links
- Bone Risks Linked to Genetic Variants
- Physical Activity Brings Lasting Bone Benefits
- New Method Builds Bone
- Osteoporosis in Aging
- Bone Health for Life
References: Cbfβ governs osteoblast-adipocyte lineage commitment through enhancing β-catenin signaling and suppressing adipogenesis gene expression. Wu M, Wang Y, Shao JZ, Wang J, Chen W, Li YP. Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):10119-10124. doi: 10.1073/pnas.1619294114. Epub 2017 Sep 1. PMID: 28864530.
Funding: NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and National Institute of Dental and Craniofacial Research (NIDCR).
