You are here
September 11, 2018
Regenerating light-sensing eye cells in mice
At a Glance
- Researchers restored some vision in mice with congenital blindness by changing supportive cells in the retina called Müller glia into light-sensing cells.
- The findings may help advance the development of regenerative therapies for blinding eye diseases.
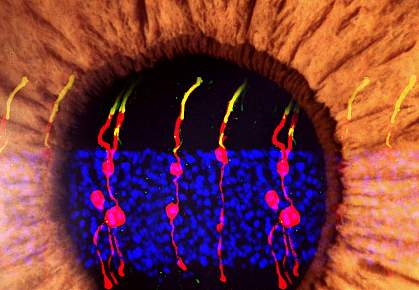
Photoreceptors are cells in the light-sensing tissue in the back of the eye called the retina. These cells absorb and convert light into electrical signals. The signals are sent to other cells in the retina and eventually to the brain, where they’re processed into the images we see. Damage to photoreceptors can lead to full or partial blindness, as in diseases like age-related macular degeneration and retinitis pigmentosa.
Photoreceptors don’t regenerate on their own in mammals. However, in some species, such as zebrafish, a supportive cell in the eye called Müller glia can divide in response to injury and turn into photoreceptors and other retinal neurons. The zebrafish can thus regain vision after severe retinal injury.
To investigate whether mammalian Müller glia cells could be coaxed into becoming photoreceptors in mammals, a team led by Dr. Bo Chen at Mount Sinai carried out a series of experiments in mice. The research was supported by NIH’s National Eye Institute (NEI). Results were published in Nature on August 23, 2018.
In the first phase of a two-stage genetic reprogramming process, Chen’s team spurred Müller glia in normal mice to divide by injecting their eyes with a gene to turn on a protein called beta-catenin. Two weeks later, they injected the eyes with factors that encouraged the newly divided cells to develop into rod photoreceptors.
The researchers used microscopy to track the newly formed cells. They found that the newly formed rod photoreceptors looked similar to normal photoreceptors.
To determine whether the Müller glia-derived rod photoreceptors were functional, the researchers tested the treatment in mice that were born blind, without functional rod photoreceptors. In addition to the treatment, they also inserted a gene to correct the mutation that caused the rods to develop defectively.
In the treated mice that were born blind, Müller glia-derived rods developed as they had in the normal mice. The researchers confirmed that the newly formed rods were communicating with other types of retinal neurons. Light-induced responses recorded from nerve cells that carry signals from photoreceptors toward the brain along with measurements of brain activity confirmed that the newly-formed rods were integrating in the visual pathway circuitry. Though the treatment restored responsiveness to light, the responses were not as strong as that of cells in normal mice.
“This is the first report of scientists reprogramming Müller glia to become functional rod photoreceptors in the mammalian retina,” says Dr. Thomas N. Greenwell, NEI program director for retinal neuroscience. “Rods allow us to see in low light, but they may also help preserve cone photoreceptors, which are important for color vision and high visual acuity. Cones tend to die in later-stage eye diseases. If rods can be regenerated from inside the eye, this might be a strategy for treating diseases of the eye that affect photoreceptors.”
Related Links
- Immune Cell Regeneration in Mouse Retina
- Genetic Engineering Prevents Retinal Cell Loss in Mice
- Novel Gene-Editing Method Improves Vision in Blind Rats
- Long-Term Benefits of Age-Related Macular Degeneration Treatments
- Refining Supplements for a Blinding Eye Disease
- The Genetics of Age-Related Macular Degeneration
- Retinal Device Restores Sight in Mice
- Age-Related Macular Degeneration
- What is Retinitis Pigmentosa?
- Your Aging Eyes: How You See as Time Goes By
References: Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B. Nature. 2018 Aug 23;560(7719):484-488. doi: 10.1038/s41586-018-0425-3. Epub 2018 Aug 15. PMID: 30111842.
Funding: NIH’s National Eye Institute (NEI); Pew Charitable Trusts; Research to Prevent Blindness; and McGraw Family Foundation for Vision Research.
