You are here
September 18, 2018
Regrowing neurons across scarred spinal tissue
At a Glance
- Researchers stimulated neurons to regrow across scarred spinal tissue in rodents using a three-pronged approach.
- The findings unveil a better understanding of the molecular underpinnings behind restoring sensation or movement after a spinal cord injury.
People can injure their spinal cord after a sudden, traumatic blow to the spine, such as a car accident or fall. Spinal cord damage can lead to a loss of sensation and/or paralysis below the injury site.
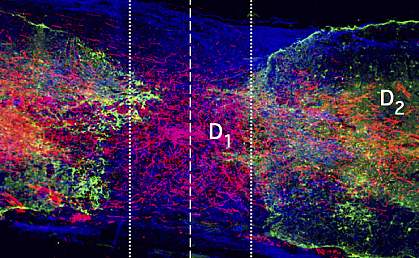
When the spinal cord is injured, a scar forms within the damaged tissue. For many years, researchers thought that the scar that forms after a spinal cord injury actively prevents damaged neurons, the cells that carry messages to and from the brain, from regrowing.
A team led by Dr. Michael V. Sofroniew at the University of California, Los Angeles, developed a three-pronged approach to regrowing neurons across scar tissue in mouse and rat models of spinal cord injury. The research was supported in part by NIH’s National Institute of Neurological Disorders and Stroke (NINDS). Results were published online in Nature on August 29, 2018.
First, the team genetically reprogrammed spinal cord neurons called propriospinal neurons in mouse and rat models of spinal cord injury. They injected viruses containing three specific genes that neurons use to grow during normal development.
The researchers next created a supportive environment for the reprogrammed neurons to grow at the injury site. They designed a gel to deliver a combination of growth-promoting proteins, which included fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF). They placed the gel at the center of the injury.
Finally, the team attempted to mimic a process in normal development in which molecules called “chemoattractants” are released as a target for growing neurons to move toward. They injected chemoattractant proteins in a trail beyond the injury site to coax the neurons to grow across the injury site.
The team found that the neurons grew robustly when all three factors were present. When any of the three treatments were omitted, minimal, if any, regrowth occurred.
Neurons traveled across the scar tissue and formed connections with neurons on the other side. The regrown neurons could conduct electrical signals across the injury site. However, this didn’t translate into an improvement in the rodents’ ability to move. This was likely because newly formed circuits usually require training before they become functional. More studies are needed to test whether the circuits could be trained to restore mobility in the rodents.
“For decades researchers have been trying to make severed neurons regrow across a spinal cord injury and reconnect with neurons on the other side. This study suggests that may require manipulating three key growth processes,” says Dr. Lyn Jakeman, program director at NINDS. “These insights are important for understanding the mechanisms of injury and regeneration that may one day be applied to develop potential treatments for spinal cord injury.”
Related Links
- Spinal Cord Stimulation Improves Hand Grip After Cervical Spinal Injury
- Spinal Cord Stimulation Helps Paralyzed People Move Hands
- Spinal Cord Injury Repair Requires Scars
- Paralyzed Men Gain Movement Without Surgery
- Paralyzed Men Regain Movement With Spinal Stimulation
- Mice Walk Again After Spinal Cord Injury
- Spinal Cord Injury
References: Required growth facilitators propel axon regeneration across complete spinal cord injury. Anderson MA, O'Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He Z, Courtine G, Sofroniew MV. Nature. 2018 Aug 29. doi: 10.1038/s41586-018-0467-6. [Epub ahead of print] PMID: 30158698.
Funding: NIH’s National Institute of Neurological Disorders and Stroke (NINDS); Dr. Miriam and Sheldon G. Adelson Medical Foundation; International Foundation for Research in Paraplegia; ALARME Foundation; Association Song Taaba; Craig H. Neilsen Foundation; European Research Council; Paralyzed Veterans Foundation of America; Swiss National Science Foundation; UCLA Broad Stem Cell Research Center; Wyss Center for Bio and Neuroengineering; and Wings for Life.
