You are here
April 16, 2024
Sensor monitors bladder fullness
At a Glance
- Researchers developed a wireless implantable device that can monitor bladder filling and emptying in real time and send data to a smartphone.
- With further development, this type of device could help monitor recovery after bladder surgery and aid patients who have compromised bladder function.

The bladder is a hollow balloon-shaped organ. It expands and contracts as needed to hold or release urine. When the bladder stretches and swells, it sends signals to the brain that it’s ready to empty. Most people take it for granted that they can control when to urinate. But for people with serious conditions like bladder cancer, paralysis, or spina bifida, the signals for bladder filling and emptying can be disrupted. This is especially true after bladder surgery.
To monitor bladder function after surgery, doctors often use a technique called urodynamic testing. The tests can be performed in a doctor’s office. But they can be uncomfortable and time-consuming. And they measure bladder function at only a single point in time.
A team led by Drs. Guillermo Ameer, John A. Rogers, and Arun Sharma at Northwestern University has been working to devise a way to continuously monitor bladder function. This type of ongoing feedback could alert doctors to abnormal or worsening bladder problems, so treatments can be adjusted. The scientists created an implantable bladder-monitoring system. They described the new system on April 2, 2024, in Proceedings of the National Academy of Sciences.
The team created an ultrathin stretchable sensor that encircles the bladder. The sensor is designed to measure strain as the bladder fills or empties. A thin wire connects the stretchable sensor to a device that attaches to the abdominal wall. This device can stream the resulting data via Bluetooth to a nearby smartphone or tablet.
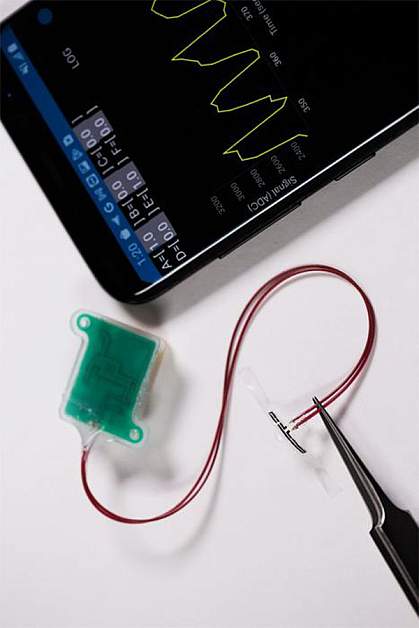
The researchers tested the monitoring system in an artificial human bladder made of silicone and other materials. This test mimicked post-surgery recovery. The sensors behaved as expected, showing that damaged and recovering regions were unable to stretch and expand as much as the “normal” bladder tissue. The researchers also demonstrated that the sensors could be created with biodegradable materials that break down in the body over time.
Rodent studies confirmed that the implantable system could detect real-time changes in bladder filling and emptying for at least 30 days. In studies of baboons, the system was able to continuously measure and monitor bladder changes for up to eight weeks.
The researchers note that the implantable bladder-monitoring system, including circuit boards and electronic components, could potentially be made entirely of biodegradable materials, as described in some earlier studies. This would avoid the need for a second surgery to remove the devices after recovery.
“The key advance here is in the development of super soft, ultrathin, stretchable strain gauges that can gently wrap the outside surface of the bladder, without imposing any mechanical constraints on the natural filling and voiding behaviors,” Rogers explains.
“This work is the first of its kind that is scaled for human use,” Ameer says. “We demonstrated the potential long-term function of the technology.”
“Our platform could provide a unique technological advance for the urological community and beyond,” adds Sharma.
The researchers continue to refine the device. More work will be needed before it could be tested in people.
—by Vicki Contie
Related Links
- Making a Wireless, Biodegradable Pacemaker
- How Your Body Senses the Urge to Urinate
- The Urinary Tract & How It Works
- Urinary Diversion
- Urodynamic Testing
References: A wireless, implantable bioelectronic system for monitoring urinary bladder function following surgical recovery. Kim J, Bury MI, Kwon K, Yoo JY, Halstead NV, Shin HS, Li S, Won SM, Seo MH, Wu Y, Park DY, Kini M, Kwak JW, Madhvapathy SR, Ciatti JL, Lee JH, Kim S, Ryu H, Yamagishi K, Yoon HJ, Kwak SS, Kim B, Huang Y, Halliday LC, Cheng EY, Ameer GA, Sharma AK, Rogers JA. Proc Natl Acad Sci U S A. 2024 Apr 2;121(14):e2400868121. doi: 10.1073/pnas.2400868121. Epub 2024 Mar 28. PMID: 38547066.
Funding: NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of Biomedical Imaging and Bioengineering (NIBIB); Michelon Family and Legacy Healthcare; National Research Foundation of Korea; Korea Health Industry Development Institute.
