You are here
August 5, 2013
Stem Cells Coaxed To Create Working Blood Vessels
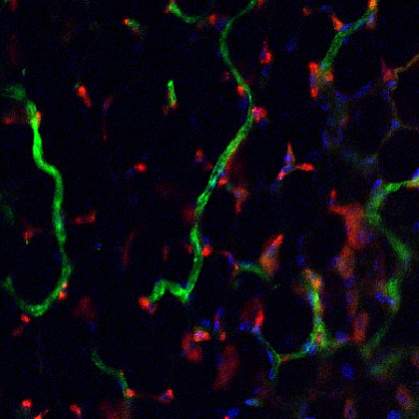
Scientists directed human stem cells to form networks of tiny blood vessels that can connect to the existing circulation in mice. The finding might assist future efforts to repair and regenerate tissues and organs, which need an adequate blood supply to grow and survive.
Blood flow is essential for just about every part of the body. Blood brings oxygen and nutrients to cells, carries away waste products and performs countless other functions. To create blood flow where it’s needed, researchers have been exploring ways to make lab-grown blood vessels that can be transplanted into the body. Earlier studies have found that the endothelial cells that line the inside of blood vessels can naturally assemble into vascular networks. But these tiny blood vessels, or microvessels, deteriorate without the presence of supporting cells called pericytes.
Some research groups are working with pluripotent stem cells, which have the potential to transform into any cell type in the body. They’ve found ways to direct the stem cells to become specific cell types, including pericytes and endothelial cells. But the process is complex, requiring multiple rounds of cell sorting and purification. Some scientists have tried growing distinct sets of precursor cells in the protein scaffolds of “decellularized” blood vessels. To date, though, many of these efforts have been inefficient and difficult to translate for clinical use.
In hopes of simplifying the process, Dr. Sharon Gerecht and her colleagues at Johns Hopkins University aimed to take advantage of the natural ability of endothelial cells to self-assemble into vascular networks. Their research was supported in part by NIH’s National Heart, Lung and Blood Institute (NHLBI) and National Cancer Institute (NCI). Their results were published in the July 30, 2013, issue of the Proceedings of the National Academy of Sciences.
The scientists first devised a way to use chemical cues to guide human pluripotent stem cells to form a specialized population made up of just 2 types of vascular cells. This bicellular population could only mature into pericytes and endothelial cells. “It makes the process quicker and more robust if you don't have to sort through a lot of cells you don't need to find the ones you do, or grow 2 batches of cells,” says Sravanti Kusuma, who developed the method.
The scientists next grew the bicellular population in a 3-D hydrogel matrix — a clear, mesh-like gel that mimics the environment inside the body. Within days, complex networks of hollow vascular tubes appeared, including both endothelial cells and pericytes.
To see how the cells would behave within the body, the scientists implanted the gel-encased microvascular networks into mice. By 2 weeks, the implanted cells and vessels had become connected to the mouse circulatory system and allowed blood flow. The researchers, though, note that additional studies are needed to fine-tune the process before it can be tested in humans.
“In demonstrating the ability to rebuild a microvascular bed in a clinically relevant manner, we have made an important step toward the construction of blood vessels for therapeutic use,” says Gerecht. “Our findings could yield more effective treatments for patients afflicted with burns, diabetic complications and other conditions in which vasculature function is compromised.”
— by Vicki Contie
Related Links
- Lab-Grown Kidneys Function in Rats
- Progress Toward an Artificial Liver Transplant
- Making a Lung Replacement
- Technique Forms Working Inner Ear Cells
References: Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):12601-6. doi: 10.1073/pnas.1306562110. Epub 2013 Jul 15. PMID: 23858432.
Funding: NIH’s National Heart, Lung and Blood Institute (NHLBI) and National Cancer Institute (NCI); American Heart Association; and National Science Foundation.
