You are here
July 23, 2019
Tracking the spread of Parkinson’s proteins from gut to brain
At a Glance
- Researchers were able to track the spread of the protein thought to cause Parkinson’s disease from the gut to the brain by way of the vagus nerve in mice.
- Results from the study suggest that finding a way to stop the spread of this protein from gut to brain might help prevent Parkinson’s disease in people.
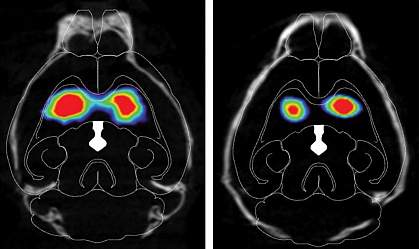
The brains of people with Parkinson’s disease contain abnormal clumps of proteins called Lewy bodies. These clumps are largely made up of the protein alpha-synuclein, which plays a role in crosstalk between brain cells. Lewy bodies interrupt this communication and are thought to be toxic to certain neurons in the brain.
These neurons produce a chemical called dopamine, which is vital for normal brain functioning. When dopamine-producing neurons start to die, the symptoms of Parkinson’s disease emerge. These include shaking, stiffness, and difficulty with walking, balance, and coordination. Some people also experience depression, anxiety, and memory loss. There is no cure for Parkinson’s disease, but treatments can provide relief from its symptoms for some time.
Abnormal clusters of alpha-synuclein have also been found in the guts of people with Parkinson’s disease. Researchers have proposed that alpha-synuclein may actually first misfold and accumulate in the gut. Recent studies showed that alpha-synuclein clumps can cause nearby normal alpha-synuclein proteins to misfold and form more clumps. These findings suggest that a chain reaction started by misfolded alpha-synuclein could travel from the gut all the way up the vagus nerve, which connects directly to the brain.
To further test this idea, researchers led by Drs. Ted Dawson and Hanseok Ko from Johns Hopkins University injected misfolded alpha-synuclein into a thin layer of muscle in the guts of mice and tracked whether it progressed to the brain. The research was funded in part by NIH’s National Institute of Neurological Disorders and Stroke (NINDS) and National Institute on Aging (NIA). Results were published on June 26, 2019, in Neuron.
The researchers found that misfolded alpha-synuclein slowly spread to regions of the mouse brain associated with Parkinson’s disease, and dopamine-producing neurons started to die. The injected mice performed worse on tests of movement, dexterity, strength, memory, and mental health than mice that hadn’t received misfolded alpha-synuclein.
The scientists also found that interfering with the chain reaction caused by the misfolded protein stopped the spread of alpha-synuclein from gut to brain. Mice that had their vagus nerve cut before injection of the misfolded alpha-synuclein into their guts did not have the protein spread to their brains. Mice engineered not to produce any normal alpha-synuclein of their own also had no signs of the misfolded protein in their brains. Both these sets of mice performed similarly on all the tests to mice that didn’t receive any misfolded alpha-synuclein.
“These findings provide further proof of the gut’s role in Parkinson’s disease, and give us a model to study the disease’s progression from the start,” Dawson says. Blocking the transmission of alpha-synuclein from gut to brain “presents a target for early intervention in the disease.”
In addition to providing further insights into Parkinson’s disease, this mouse system could allow researchers to test treatments that might prevent or halt disease progression.
—by Sharon Reynolds
Related Links
- Appendix Linked to Toxic Parkinson’s Protein
- Gut Communicates Directly with Brain
- Tracking Parkinsonian Disease Progression
- Parkinson’s Disease Information Page
- Parkinson’s Disease (Aging)
- Parkinson’s Disease: Understanding a Complicated Condition
References: Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson's Disease. Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Neuron. 2019 Jun 26. pii: S0896-6273(19)30488-X. doi: 10.1016/j.neuron.2019.05.035. [Epub ahead of print]. PMID: 31255487.
Funding: NIH’s National Institute of Neurological Disorders and Stroke (NINDS) and National Institute on Aging (NIA); JPB Foundation; Adrienne Helis Malvin Medical Research Foundation.
