You are here
February 9, 2016
Visualizing a cancer drug target at atomic resolution
At a Glance
- Using cryo-electron microscopy, researchers were able to view, in atomic detail, the binding of a potential small molecule drug to a key protein in cancer cells.
- The results illustrate how the imaging technique can help advance drug development.
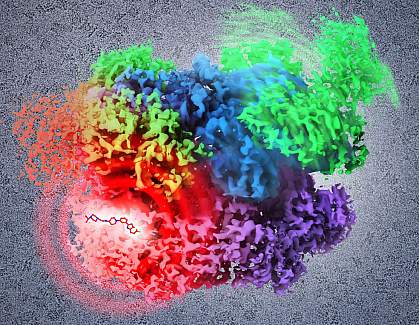
Determining the 3-D structure of a protein at a fine level of detail is important for drug development. Understanding how the drug and protein interact at an atomic level can allow scientists to design new drugs to alter the target protein’s function.
A research team led by Dr. Sriram Subramaniam of NIH’s National Cancer Institute (NCI) has been using an imaging technique called cryo-electron microscopy (cryo-EM) to determine atomic structures. Protein suspensions are flash-frozen at very low temperatures to quickly stabilize the water around proteins without allowing damaging ice crystals to form. The suspensions are then bombarded with electrons to capture images. The researchers computationally combine thousands of these 2-D images of the molecules in different orientations to produce 3-D structures. Cryo-EM has been gaining in popularity because it enables the visualization of specimens under near-native, or natural, conditions.
The scientists have used cryo-EM to study a variety of molecules, including proteins and receptors found in brain cells. In their latest study, the team visualized the binding of a potential small molecule drug to a key enzyme in cancer cells. The enzyme, p97, is critical for protein quality control in the cell and a potential anti-cancer target. The work appeared online on January 28, 2016, in Science.
Using cryo-EM, the researchers were able to image full-length p97 at an overall resolution of 2.3 Å, which is much finer than previous structural studies of p97 had achieved. The researchers were also able to establish, at atomic resolution, the sequence of structural changes that normally occur in the protein.
The scientists determined the mode of binding and contact sites of a small molecule inhibitor of p97 activity. Rather than sitting in an active site binding pocket of the protein, the inhibitor wedges between the upper and lower halves of the protein. With the inhibitor in place, the protein can no longer undergo its normal ratchet-like rotational motion, instead locking up in a single position. These deductions about inhibitor action were only possible because cryo-EM was able to capture the whole protein in multiple conformations, or forms.
“Our latest research provides new insights into the protein structures and interactions that are critical for the activity of a cancer cell, and this knowledge will hopefully enable the design of clinically useful drugs,” Subramaniam says.
“Cryo-EM is positioned to become an even more useful tool in structural biology and cancer drug development,” says Dr. Douglas Lowy, acting director of NCI. “This latest finding provides a tantalizing possibility for advancing effective drug development.”
Related Links
- High-Resolution Imaging Technique May Advance Drug Design
- Structural States of a Brain Receptor Revealed
- Key HIV Protein Structure Revealed
References: 2.3 Å resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition. Banerjee S, Bartesaghi A, Merk A, Rao P, Bulfer SL, Yan Y, Green N, Mroczkowski B, Neitz RJ, Wipf P, Falconieri V, Deshaies RJ, Milne JL, Huryn D, Arkin M, Subramaniam S. Science. 2016 Jan 28. pii: aad7974. [Epub ahead of print]. PMID: 26822609.
Funding: NIH’s National Cancer Institute (NCI).
