You are here
March 31, 2014
Why Many Vein Grafts Fail
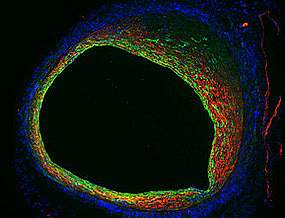
Researchers identified a biological pathway that contributes to vein graft failure following bypass surgery. The finding points to potential drugs that might help to reduce such failures.
Bypass surgery is a common procedure in the United States. A healthy artery or vein from elsewhere in the body is grafted onto arteries that feed the heart to bypass clogged vessels and restore blood flow. The great saphenous vein—the large vein running up the length of the leg—is often used as a bypass due to its size and the ease of removing a small segment.
After grafting, implanted veins remodel to become more arterial. However, the remodeling can go awry and the vein can become too thick, resulting in clogged blood flow. About 40% of vein grafts fail within 18 months of the operation.
To better understand how this process goes awry, a team led by Dr. Manfred Boehm of NIH’s National Heart, Lung, and Blood Institute (NHLBI) examined veins from mouse models of bypass surgery. The scientists suspected that a process known as endothelial-to-mesenchymal transition, or EndoMT, may cause the inside of the vein to over-thicken. During EndoMT, endothelial cells that line the inner surface of the vein proliferate and convert into more fibrous and muscle-like cells. These mesenchymal cells begin to accumulate on the inner wall, narrowing the vessel. The inner walls of the vein become too thick, slowing or blocking the blood flow that the graft was intended to restore.
To study this process, the scientists used mice in which endothelial cells express a protein that can be used to track their fate. The results were published on March 12, 2014, in Science Translational Medicine.
By tracing the fate of endothelial cells, the researchers showed that endothelial-derived cells contribute to vein thickening through EndoMT. They discovered that the process was triggered by transforming growth factor beta (TGF-beta), a secreted protein known to control the proliferation and maturation of several cell types.
TGF-beta, the scientists found, becomes highly expressed just a few hours after graft surgery. Inhibition of the TGF-beta signaling pathway reduced overgrowth in the grafted veins.
The team examined human veins taken from failed bypass operations and found corroborating evidence for a role for EndoMT in human graft failure. In short-term grafts (less than 1 year), many of the cells inside the human veins displayed both endothelial and mesenchymal cell characteristics, while in long-term grafts (more than 6 years) the cells on the inner wall were primarily mesenchymal in nature.
“This study shows for the first time that endothelial cells in the vein directly contribute to blood vessel narrowing following a vein graft,” Boehm says. “Now that we better understand the mechanism that causes the abnormal thickening, we can look for therapeutic strategies to attenuate it and reduce the number of bypass reoperations we need to perform each year.”
Certain drugs that are known to inhibit TGF-beta are a possible treatment strategy. However, more studies will be needed before any clinical studies can begin.
Related Links
References: TGF-β Signaling Mediates Endothelial-to-Mesenchymal Transition (EndMT) During Vein Graft Remodeling. Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, Boehm M. Sci Transl Med. 2014 Mar 12;6(227):227ra34. doi: 10.1126/scitranslmed.3006927. PMID: 24622514.
Funding: NIH’s National Heart, Lung, and Blood Institute (NHLBI); the Commission on Higher Education; the Philippine Council on Advanced Science and Technology Research and Development; Department of Science and Technology (PCASTRD-DOST) in the Philippines; an NIH Marshall Scholarship; and Boehringer Ingelheim Fonds.
