You are here
October 31, 2017
Hydrogel-grown tissues speed wound healing in mouse colon
At a Glance
- Researchers designed an injectable hydrogel that grew intestine-like tissues and accelerated wound healing in the intestines of mice.
- These findings suggest new treatments to explore for intestinal injuries caused by diseases such as inflammatory bowel disease.
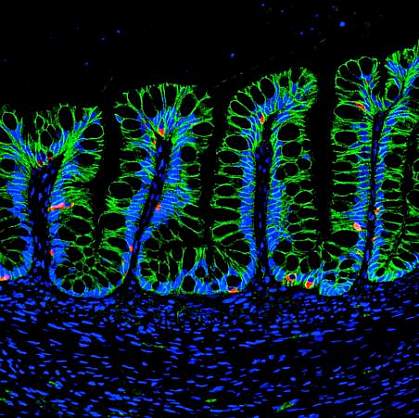
Engineered organ-like structures, called organoids, have the potential to repair or replace tissue that is damaged or diseased. Organoids are grown by bringing together stem cells, scaffolds made of biomaterials, and biologically active molecules. With the right mixture, these can combine to assemble functional tissues.
To engineer specific tissues, scaffolds must contain the proper combination of proteins, stem cells, and growth factors. Scaffold materials must also be carefully formulated to minimize potential toxic effects.
Matrigel is a commonly used scaffold that is derived from animal cells. This gel presents clinical challenges, though, because it could potentially transfer animal diseases. Hydrogels are a synthetically made, flexible material that can also serve as a scaffold and may be safer for clinical use.
A research team led by Dr. Andrés J. García at Georgia Institute of Technology and Drs. Asma Nusrat and Jason Spence at the University of Michigan compared hydrogel-grown versus Matrigel-grown intestine-like organoids in mice. The study was funded in part by NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and other NIH components. Results were published online on October 23, 2017 in Nature Cell Biology.
The team first created hydrogels using different chemical formulations of polymers to see which supported the growth of human intestinal organoids in the lab. They then compared the hydrogel that best supported organoid growth with Matrigel. Organoid growth and proliferation were similar between the two scaffolds.
Hydrogel-grown and Matrigel-grown human intestinal organoids were then implanted into the kidneys of mice. Both developed, or differentiated, into tissue that resembled mature human intestine after twelve weeks.
The hydrogel was formulated as a solution that could be injected before solidifying. This allowed the gel to be delivered using an endoscope, a flexible tube and camera that can be inserted into the intestine. The researchers injected the hydrogel into wounded colons of mice. The gel engrafted to the intestine, where intestine-like tissue developed and helped heal the wound.
“In this work, we demonstrated that the hydrogels facilitate the transplantation of [human intestinal organoids] into an injured intestine, suggesting that this technique has significant implications for treating intestinal injuries caused by diseases such as inflammatory bowel disease,” Nusrat explains.
“This work provides a proof of principle for using stem cell-derived human intestinal organoids in a therapeutic setting.” García says.
—Tianna Hicklin, Ph.D.
Related Links
- Stem Cells Grown on Scaffold Mimic Hip Joint Cartilage
- Stem Cells Coaxed To Create Working Blood Vessels
- Liver Stem Cells Discovered in Mice
- Fixing Flawed Body Parts
- Better Check Your Bowels
- Grumbling Guts?
- Irritable Bowel Syndrome
- Tissue Engineering and Regenerative Medicine
- Stem Cell Basics
References: Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, García AJ. Nat Cell Biol. 2017 Oct 23. doi: 10.1038/ncb3632. [Epub ahead of print] PMID: 29058719.
Funding: NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), and National Center for Advancing Translational Sciences (NCATS); Regenerative Engineering and Medicine Research Center between Emory University, Georgia Tech, and the University of Georgia; National Science Foundation; Alfred P. Sloan Foundation; Crohn’s and Colitis Foundation of America; and the János Bolyai Research Fellowship.
