You are here
RADx Programs
On this page
RADx® Tech
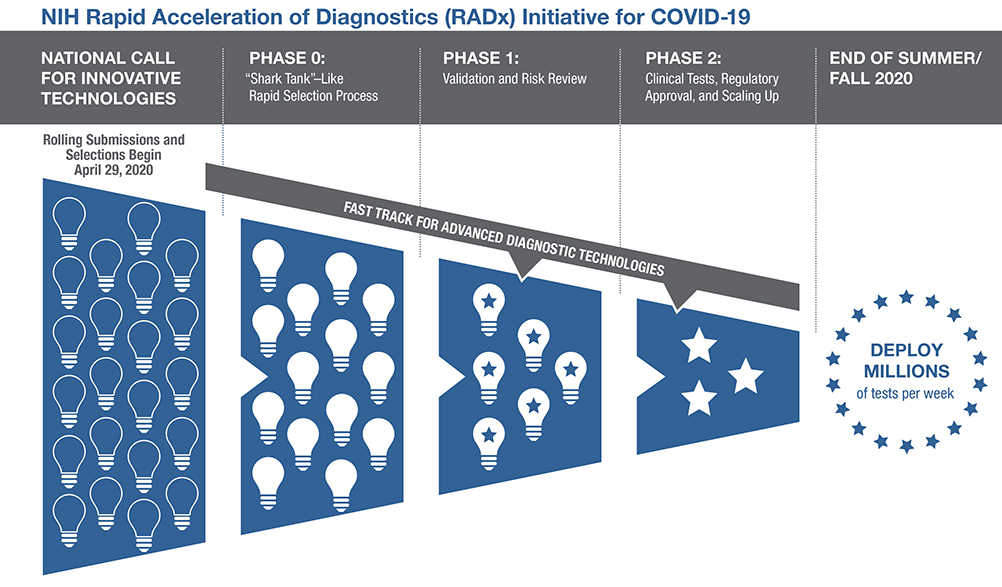
The RADx Tech initiative aims to speed the development, validation, and commercialization of innovative point-of-care and home-based tests, as well as improve clinical laboratory tests, that can directly detect the virus.
(Click image to enlarge)
The RADx Tech initiative aims to speed the development, validation, and commercialization of innovative point-of-care and home-based tests, as well as improve clinical laboratory tests, that can directly detect the virus. RADx Tech has expanded the Point-of-Care Technologies Research Network (POCTRN) established several years ago by NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB). The network is using a flexible, rapid process to infuse funding and enhance technology designs at key stages of development, with expertise from technology innovators, clinical testing, regulatory affairs, entrepreneurs, and business leaders.
Scientists and inventors with a rapid testing technology compete in a national “shark tank”-type COVID-19 testing challenge for a share of up to $500 million over all phases of development. The technologies go through a highly competitive, rapid three-phase selection process to identify the best candidates for at-home or point-of-care tests for COVID-19. Finalists are then matched with technical, business, and manufacturing experts to increase the odds of success.
If certain selected technologies are already relatively far along in development, they can be advanced immediately to the appropriate step in the commercialization process.

RADx® Underserved Populations (RADx-UP)
The overarching goal of the RADx-UP initiative is to understand the factors associated with disparities in COVID-19 morbidity and mortality and to lay the foundation to reduce disparities for those underserved and vulnerable populations who are disproportionately affected by, have the highest infection rates of, and/or are most at risk for complications or poor outcomes from the COVID-19 pandemic.
NIH is developing community-engaged projects across the United States to assess and expand COVID-19 testing for these underserved and/or vulnerable populations, which include health disparity populations, particularly African Americans and American Indians/Alaska Natives; those in nursing homes, jails, rural areas, or underserved urban areas; pregnant women; and the homeless.
Specific activities include:
- Establishing multiple clinical research sites across the country to evaluate, in real-time, a variety of testing methods in specific populations, areas, and settings
- Encouraging collaboration between the program sites and the community — tribal health centers, houses of worship, homeless shelters, and prison systems — to identify and address their unique needs
- Developing testing strategies to apply technological advances emerging from the various RADx efforts in real-world settings, examples include the “Say Yes! COVID Test” and Return to School testing initiatives
RADx-UP will develop testing strategies to apply the technological advances emerging from the various RADx efforts in real-world settings, such as distributing home diagnostic kits.
The program is occuring in two phases. The first phase focuses on communities with established research infrastructures and partnerships to understand COVID-19 testing patterns, and implement strategies or interventions with the potential to rapidly increase reach, access, acceptance, uptake, and sustainment of FDA-authorized/approved diagnostics among vulnerable populations in geographic locations that are underserved. With extensive investment in the development and validation of new testing technologies, NIH anticipates significant changes in the landscape of testing and diagnostic approaches, as well as shifts in the pandemic itself over the next several months. Phase II of the RADx-UP initiative will be released at a later date to address developments for future community-engaged research.

RADx® Radical (RADx-rad)
RADx-rad will support new, non-traditional approaches, including rapid detection devices and home-based testing technologies, that address current gaps in COVID-19 testing. The program will also support new or non-traditional applications of existing approaches to make them more usable, accessible, or accurate. These may lead to new ways to identify the current SARS-CoV-2 virus as well as potential future viruses.
Examples of non-traditional approaches include:
- Community wastewater analysis and other surveillance methods, particularly those focused on high risk population, to identify the virus and measure the spread of infection
- Novel analytical platforms, such as next generation sequencing, coupled with point-of-care, non-invasive sample collection
- Screening and home-based tests that detect in sensory or other functions to predict disease at early onset
- Integration of artificial intelligence (AI) systems with novel biosensing or laboratory diagnostics with digital health technologies for screening, diagnosing, and monitoring COVID-19, including in children, and to predict disease severity
- Automatic, real-time detection and tracing of SARS-COV-2 by integrating virus-sensing elements with touchscreen or other digital devices
RADx® Advanced Technology Platforms (RADx-ATP)
The RADx-ATP program seeks to increase testing capacity and throughput by identifying existing and late stage testing platforms for COVID-19 that are far enough advanced to achieve rapid scale-up or expanded geographical placement in a short amount of time. These efforts will focus on scaling up technologies, including improving existing high-throughput platforms, to increase performance.
Specific RADx ATP project examples include:
- Scale up of existing technologies that are already or near FDA authorization for SARS-CoV-2 detection. Some emphasis will be placed on differential testing to distinguish SARS-CoV-2 vs. Influenza virus detection
- Establish or expand regional testing hubs, to validate and perform clinical tests. This could also be supportive of identifying needs for facilities to expand testing in underserved populations
- Accelerating the development and implementation of tests for multiple infectious diseases, including viruses and the immune response, bacteria, and genetics
Starting in summer 2020, this two-year effort will award funding on a rolling basis.

RADx® Data Hub
The RADx® Data Hub is a centralized data repository that provides access to analytic tools and de-identified COVID-19 data from the RADx Initiative.
The RADx Data Hub supports efforts to understand COVID-19 and factors associated with disparities in COVID-19 morbidity and mortality in underserved and vulnerable populations. To do this, the RADx Data Hub seeks to accelerate scientific solutions and innovations in the development, commercialization, and implementation of technologies for COVID-19 testing by providing de-identified COVID-19-related data, algorithms, and other capabilities generated by various digital health solutions and technologies.
The RADx Data Hub enables researchers to:
- Query a wide range of health data, including clinical, behavioral, social determinants of health, survey, interview, diagnostic and testing results, viral sequences, output from smart sensors, self-reported symptoms, and imaging data
- Collaborate with other members of the research community by sharing the results of analyses and other relevant data
- Make use of AI machine learning algorithm research
This page last reviewed on August 13, 2024