You are here
June 7, 2016
Alzheimer’s protein may have natural antibiotic role
At a Glance
- Beta-amyloid, a protein implicated in Alzheimer’s disease, appears to help protect mice and worms from infection with bacteria or fungi.
- The results suggest that Alzheimer’s disease may result from certain infections.
- If validated, the findings could lead to novel approaches to prevent the disease.
Alzheimer’s disease is a progressive neurodegenerative disorder that damages healthy brain cells. It causes the brain’s nerve cells to lose their ability to function and communicate with each other, and eventually die. Over time, this process destroys memories, causes personality changes, and makes it hard to perform daily activities.
One of the major hallmarks of Alzheimer’s disease is amyloid plaques that are found in between nerve cells. These plaques are made up of a small protein chain called beta-amyloid, along with other proteins and pieces of nerve cells. Beta-amyloid is believed to result from the abnormal processing of the amyloid precursor protein (APP). The biological purpose of beta-amyloid hasn’t been known.
Beta-amyloid has some features similar to a family of immune molecules called antimicrobial peptides, or AMPs. A team co-led by Drs. Robert Moir and Rudolph Tanzi at Massachusetts General Hospital and Harvard Medical School investigated whether beta-amyloid might also have antimicrobial properties. The research was funded in part by NIH’s National Institute of Allergy and Infectious Diseases (NIAID). Results were published in Science Translational Medicine on May 25, 2016.
The team compared how bacterial infections progressed in the brains of genetically modified mice that either had too much or no beta-amyloid. Mice with excess beta-amyloid survived longer than the controls and had less bacteria in their brains. Mice lacking beta-amyloid (from the genetic removal of APP) died more often from infection.
Beta-amyloid also showed protective effects in C. elegans worms that were genetically altered to express human beta-amyloid. These worms lived longer than controls after being infected with the Candida albicans fungus. Cultured human brain cells infected with this fungus also had higher survival rates when they were altered to produce more beta-amyloid.
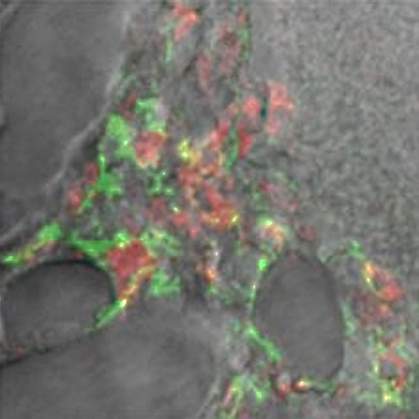
Experiments with fungal cells showed that beta-amyloid disrupted Candida’s ability to adhere directly to host cells—a mechanism necessary for infection. The microbes also clumped together in the presence of beta-amyloid. Both effects are similar to those seen with antimicrobial peptides. Further work revealed other similarities to AMP activity.
Electron microscopy showed that the microbes were entangled in beta-amyloid fibers extending from the surfaces of the fungal cells, suggesting an entrapment role for beta-amyloid. Electron microscopy of the mouse brains infected with bacteria also showed that the microbes were embedded in beta-amyloid fibers. The authors propose that beta-amyloid’s role in Alzheimer’s disease may result from dysregulation of normal immune system activity rather than abnormal processing of APP as previously believed.
“Our findings raise the intriguing possibility that Alzheimer’s pathology may arise when the brain perceives itself to be under attack from invading pathogens, although further study will be required to determine whether or not a bona fide infection is involved,” Moir says. “If validated, our data also warrant the need for caution with therapies aimed at totally removing beta-amyloid plaques. Amyloid-based therapies aimed at dialing down but not wiping out beta-amyloid in the brain might be a better strategy.”
—by Tianna Hicklin, Ph.D.
Related Links
- Human Cells Model Alzheimer’s Disease
- New Genes Tied to Alzheimer’s Disease
- Study Points to Possible Blood Test For Memory Decline, Alzheimer’s
- Clues to Alzheimer’s Disease
- Dealing with Dementia
- Can We Prevent Alzheimer’s Disease?
- NIHSeniorHealth: Alzheimer's Disease
- Alzheimer’s Disease Education and Referral (ADEAR) Center
- Alzheimers.gov
References: Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. Sci Transl Med. 2016 May 25;8(340):340ra72. doi: 10.1126/scitranslmed.aaf1059. PMID: 27225182.
Funding: NIH’s National Institute of Allergy and Infectious Disease (NIAID); Cure Alzheimer’s Fund; and Helmsley Charitable Trust.
